Senescence Phenomena and Metabolic Alteration in Mesenchymal Stromal Cells from a Mouse Model of Rett Syndrome
Squillaro T, Alessio N, Capasso S, Di Bernardo G, Melone MAB, Peluso G, Galderisi U
Abstract: Chromatin modifiers play a crucial role in maintaining cell identity through modulation of
gene expression patterns. Their deregulation can have profound effects on cell fate and functions.
Among epigenetic regulators, the MECP2 protein is particularly attractive. Mutations in the Mecp2 gene
are responsible for more than 90% of cases of Rett syndrome (RTT), a progressive neurodevelopmental
disorder. As a chromatin modulator, MECP2 can have a key role in the government of stem cell biology.
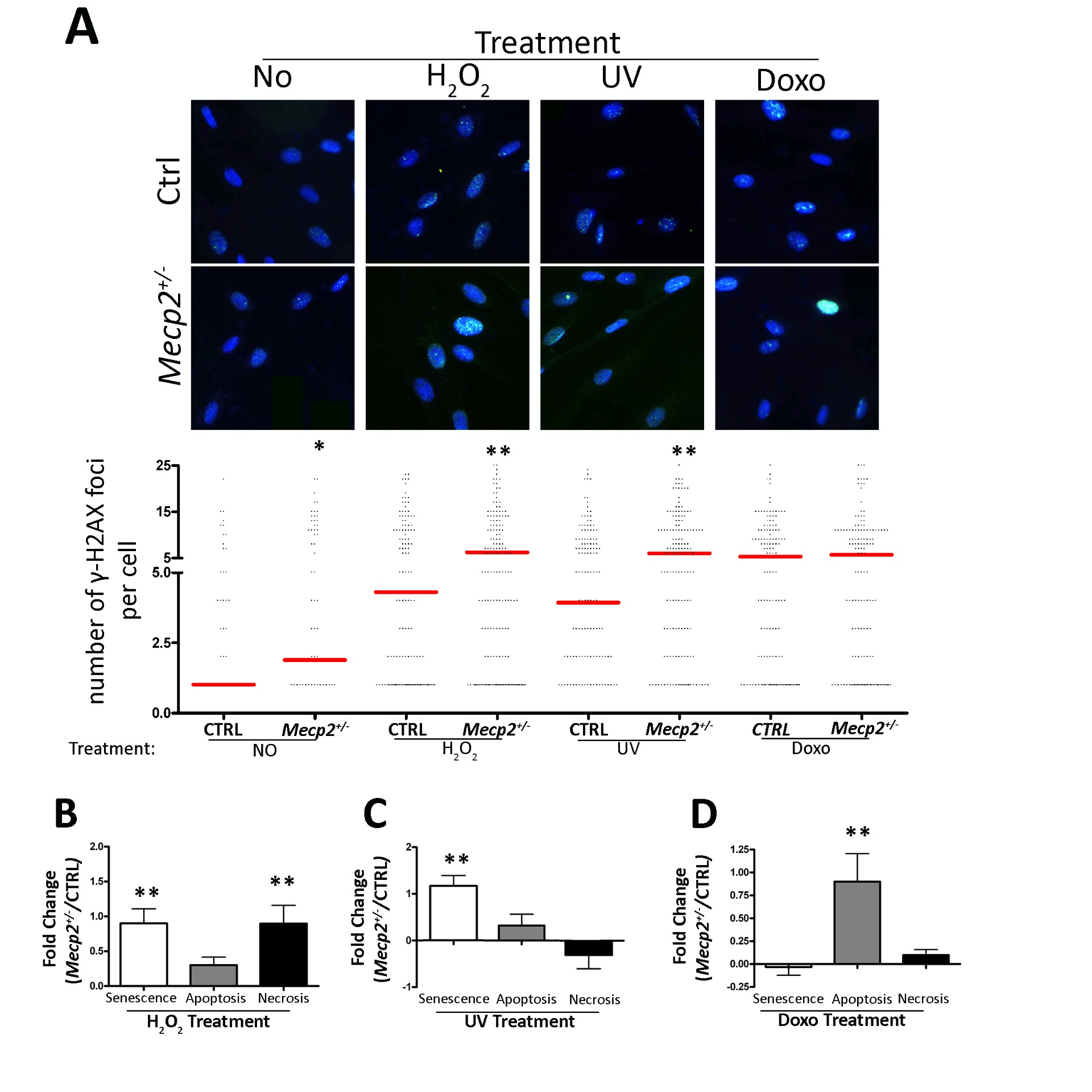
Previously, we showed that deregulated MECP2 expression triggers senescence in mesenchymal stromal
cells (MSCs) from (RTT) patients. Over the last few decades, it has emerged that senescent cells show
alterations in the metabolic state. Metabolic changes related to stem cell senescence are particularly
detrimental, since they contribute to the exhaustion of stem cell compartments, which in turn determine
the falling in tissue renewal and functionality. Herein, we dissect the role of impaired MECP2 function in
triggering senescence along with other senescence-related aspects, such as metabolism, in MSCs from a
mouse model of RTT. We found that MECP2 deficiencies lead to senescence and impaired mitochondrial
energy production. Our results support the idea that an alteration in mitochondria metabolic functions
could play an important role in the pathogenesis of RTT.